Researchers from the RIKEN Cluster for Pioneering Research have used an advanced method to show that a chemical reaction energized by light occurs 10,000 times quicker at the air-water interface—what is generally called the water surface—compared to the bulk of the water, even when the light has similar energy.
This discovery could help gain better insights into the several essential biological and chemical processes that occur at the water surface.
Water is the most essential liquid present in nature, and the study has demonstrated that there is indeed something unique about the interface. For reasons not clearly understood, it seems that a few chemical reactions occur readily when the molecules are partially in the water, but not when dissolved completely.
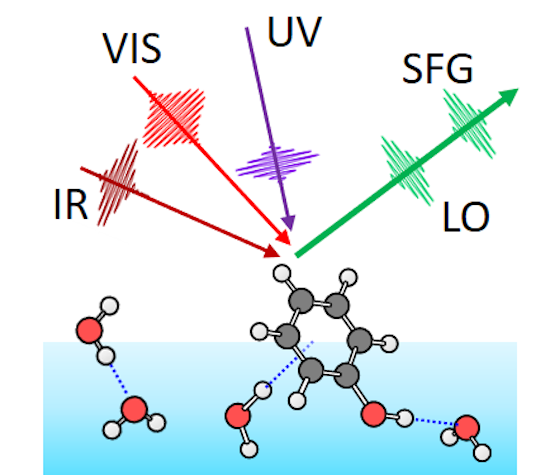
One problem blocking the insight is that how chemical reactions progress at the interface is not clearly understood. To analyze this, the RIKEN group employed an advanced method known as ultra-fast phase-sensitive interface-selective vibrational spectroscopy.
Although it is hard to explain, it typically implies that one can get a high speed movie of the intermediate molecules formed as a chemical reaction occurs at an interface. In this instance, 'high-speed' denotes around 100 fs, or less than a trillionth of a second.
With the help of this technique, they examined the photoionization of phenol, a reaction that has been analyzed well in bulk water, with the help of high speed pulses of ultraviolet light. The experiments demonstrated that the same reaction occurred at the interface but because of discrepancies in the conditions there, the reaction occurred approximately 10,000 times quicker.
It was exciting to find that the reaction speed for phenol is so phenomenally different, but in addition, our method for directly observing chemical reactions at the water surface in real time could also be applied to other reactions, and could help us get a better understanding of how reactions proceeds in this special environment.
Satoshi Nihonyanagi, Study Author, RIKEN
The study was published in the Nature Chemistry journal.
The fact that the there is a 10,000-fold difference in the reaction rate of a basic organic molecule such as phenol between the bulk water and the water surface is also very important for catalytic chemistry, the field of study that aims to promote and control chemical reactions.
Tahei Tahara, Leader of Research Group, RIKEN
“In addition, water in nature exists as seawater, which has bubbles and aerosols, thus having a vast surface area. Our work could help us to understand how molecules are adsorbed on the surface of water, leading to chemical reactions that have an enormous impact on the global environment,” Tahara concluded.
Journal Reference:
Kusaka, R., et al. (2021) The photochemical reaction of phenol becomes ultrafast at the air-water interface. Nature Chemistry. doi.org/10.1038/s41557-020-00619-5.
Source: https://www.riken.jp/en/
"occur" - Google News
February 16, 2021 at 11:11PM
https://ift.tt/3qrnjDP
Chemical Reactions Occur 10,000 Times Faster at Air-Water Interface - AZoM
"occur" - Google News
https://ift.tt/2UoDqVw
https://ift.tt/2Wq6qvt
Bagikan Berita Ini















0 Response to "Chemical Reactions Occur 10,000 Times Faster at Air-Water Interface - AZoM"
Post a Comment